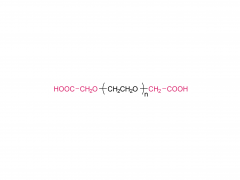
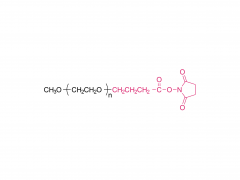
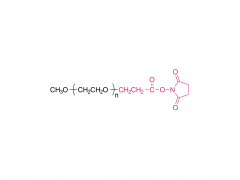
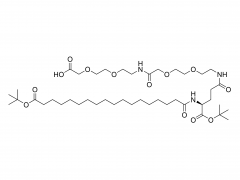
ACS Biomater Sci Eng. 2019 Nov 11;5(11):5759-5769. doi: 10.1021/acsbiomaterials.9b00218. Epub 2019 Apr 24. N-Hydroxysuccinimide Bifunctionalized Triblock Cross-Linker Having Hydrolysis Property for a Biodegradable and Injectable Hydrogel System Abstract The design of biocompatible, degradable, and injectable hydrogel has been attractive for achievement of safe and efficient tissue engineering. Herein, we designed a N-hydroxysuccinimide (NHS) ester-terminated ABA triblock copolymer composed of poly(ethylene glycol) (PEG) as hydrophilic A segments and poly(dl-lactide) (PLA) as B segment having hydrolysis property (NHS-PEG-b-PLA-b-PEG-NHS) to be a cross-linker of polymer segments having amine groups for facile construction of injectable and degradable hydrogel. The PLA domain, which is widely accepted hydrolyzable segments, is inherently hydrophobic and simple introduction of the NHS group on the ends of PLA would not have high reactivity in aqueous milieu to construct injectable hydrogel. Thus, in this design, hydrophilic PEG was introduced as A segments to increase the reactivity of NHS groups at the ends of linkers by increasing the mobility. To demonstrate the property as a cross-linker for constructing degradable and injectable hydrogel, carboxylmethyl chitosan (CH), which is a polymer segment having amine groups, and NHS-PEG-b-PLA-b-PEG-NHS solutions were mixed to form the hydrogel (CH/PEG-PLA-PEG) under physiological condition. The formation of CH/PEG-PLA-PEG hydrogel proceeded within minute-order period after mixing the solutions, suggesting NHS-PEG-b-PLA-b-PEG-NHS is applicable to the cross-linker for construction of injectable hydrogel system with time-dependent gelation property. Degradation of the obtained CH/PEG-PLA-PEG hydrogel was observed, whereas that of CH/PEG, which was prepared from NHS-PEG-NHS and CH, was not observed, appealing the degradation property of the CH/PEG-PLA-PEG hydrogel based on hydrolysis of the PLA domain. Furthermore, chondrocytes embedded in CH/PEG-PLA-PEG hydrogels promoted collagen synthesis compared to CH/PEG. These demonstrations indicate the designed NHS-PEG-b-PLA-b-PEG-NHS is a promising cross-linker to construct the injectable and degradable hydrogel and eventually promote hydrogel performance as a tissue regeneration scaffold. Keywords: biodegradable and injectable hydrogel; chitosan; poly(dl-lactide); poly(ethylene glycol); tissue engineering. For more product information, please contact us at: US Tel: 1-844-782-5734 US Tel: 1-844-QUAL-PEG CHN Tel: 400-918-9898 Email: sales@sinopeg.com
View More