Introduction
Lipid nanoparticles (LNPs) represent the core technological platform for mRNA drug delivery, enabling efficient nucleic acid transport by mimicking cellular membrane structures. This article provides a professional analysis of LNP composition, key steps in transmembrane delivery, and underlying molecular mechanisms.
I. Composition and Structural Characteristics of LNPs
LNPs consist of the following core components:
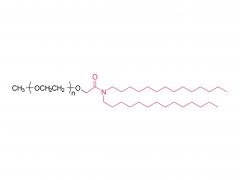
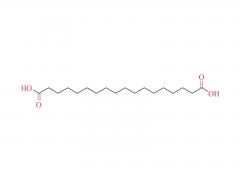
1. Cationic/Ionizable Lipids (e.g., SM-102, ALC-0315)
- Function: Positively charged under acidic conditions to bind negatively charged mRNA via electrostatic interactions; neutral at physiological pH to reduce toxicity.
- Critical Role: Drive LNP self-assembly into a core-shell structure encapsulating mRNA.
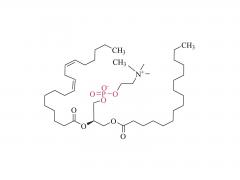
2. Helper Lipids (e.g., DSPC, cholesterol)
- DSPC (1,2-Distearoyl-sn-glycero-3-phosphocholine): Enhances membrane stability and promotes fusion with target cell membranes.
- Cholesterol: Modulates lipid bilayer fluidity and improves serum stability of LNPs.
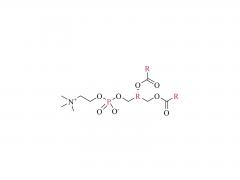
3. PEGylated Lipids (e.g., DMG-PEG2000)
- Function: Reduce LNP aggregation via steric hindrance, prolong circulation time, and regulate particle size (typically 60–100 nm).
II. Transmembrane Delivery Process of LNP-Encapsulated mRNA
1. Cellular Targeting and Endocytosis
- Targeting Mechanisms: LNPs accumulate in target cells (e.g., muscle cells, immune cells) via passive targeting (enhanced permeability and retention, EPR effect) or active targeting (ligand modification).
-Endocytic Pathways: LNPs enter cells via clathrin-mediated endocytosis or caveolae-dependent uptake, forming early endosomes.
2. Endosomal Escape
- pH-Sensitive Mechanism:
- Endosomal acidification (pH 5.0–6.0) triggers protonation of ionizable lipids, inducing LNP structural reorganization.
- Cationic lipids interact with anionic phospholipids (e.g., phosphatidylserine) in endosomal membranes, disrupting membranes via the "proton sponge effect" or membrane fusion to release mRNA into the cytoplasm.
3. Cytoplasmic Release and Translation of mRNA
- Release Mechanism: Following lipid membrane disintegration, mRNA diffuses or is chaperoned into ribosomes.
- Translation Initiation: Free mRNA utilizes eukaryotic translation initiation factors (eIF4E/eIF4G complexes) to synthesize proteins (e.g., SARS-CoV-2 spike protein in vaccines).
III. Key Molecular Mechanisms and Optimization Strategies
1. Enhancing Endosomal Escape Efficiency
- Use lipids with unsaturated tail chains (e.g., DLin-MC3-DMA) to improve membrane fusion.
- Incorporate pH-sensitive peptides (e.g., GALA peptide) to assist membrane disruption.
2. Reducing Immunogenicity
- Optimize PEGylated lipid ratios to avoid accelerated blood clearance (ABC phenomenon).
- Purify LNPs to remove free mRNA, minimizing TLR7/8 activation risks.
3. Tissue-Specific Delivery
- Adjust LNP surface charge (near-neutral ζ-potential) for hepatocyte targeting.
- Modify LNPs with GalNAc ligands to target hepatic asialoglycoprotein receptors (ASGPR).
IV. Technical Challenges and Future Directions
Challenges:
- Low delivery efficiency to lungs, brain, and other tissues.
- Potential anti-PEG antibody induction with repeated dosing.
Advances:
- Develop biodegradable lipids (e.g., ester-modified lipids) to reduce toxicity.
- Leverage AI for novel lipid library design and LNP optimization.
Conclusion
The transmembrane delivery of mRNA by LNPs relies on precise physicochemical and cellular biological interactions, particularly dynamic lipid-membrane regulation. While LNP technology has propelled mRNA vaccines and gene therapies into clinical translation, ongoing optimization of delivery precision and long-term safety remains critical.
References
1. Hou, X. et al. (2021). *Nat. Rev. Mater.* **6**, 1078–1094.
2. Cullis, P.R. & Hope, M.J. (2017). *Mol. Ther.* **25**, 1467–1475.