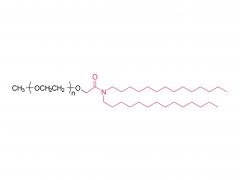
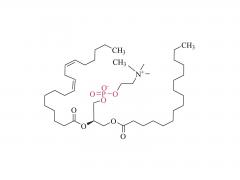
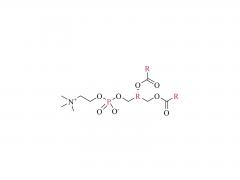
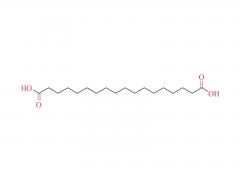
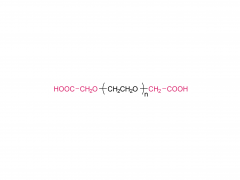
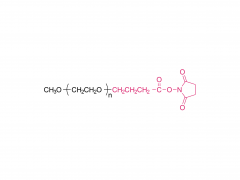
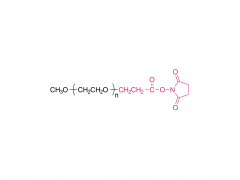
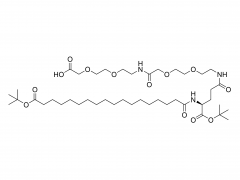
Bioorg Med Chem Lett. 2018 May 1;28(8):1363-1370. doi: 10.1016/j.bmcl.2018.03.005. Epub 2018 Mar 3. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG Abstract Kadcyla® (T-DM1), an antibody-drug conjugates (ADCs) for HER2+ breast cancer treatment, has been approved by the Food and Drug Administration (FDA) in 2013. An ADC of random lysine conjugation, it has difficulties in DAR control and unsatisfactory PK due to uneven DAR distribution. It also gives rise to aggregation during conjugation because of the hydrophobicity nature of the cytotoxin, DM1. The linker-drug in T-DM1, SMCC-DM1 is hydrophobic and requires certain percentage of organic solvent such as DMA in the conjugation solution, limiting the manufacturing process in an organic-solvent-compatible device and adding extra costs. To address these problems, a site-specific conjugation method was developed involving full reduction of antibody and full conjugation with the bridge-like conjugator-drug, based on the work of Caddick and co-workers, to obtain a site-directed antibody-drug conjugate with DAR 4. The bridge-like conjugator was assembled with SMCC-DM1 and different lengths of hydrophilic polyethylene glycol (PEG) moiety. By applying PEG moiety in the side chain of the linker-drug, the organic solvent used in the conjugation can be reduced. When the PEG length is about 26 units, organic solvent is no longer needed in the conjugation. Reducing the amount of organic solvent in conjugation could also diminish the aggregation occurrence during the conjugation. Moreover, the conjugation configuration with the designed conjugator was also discussed in the article. The binding affinity of the resulting ADCs did not show significant decrease and the cell based assay and animal study have shown the comparable results with T-DM1. Keywords: Antibody-drug conjugates; Maytansine DM1; PEGlyation; Site-specific. For more product information, please contact us at: US Tel: 1-844-782-5734 US Tel: 1-844-QUAL-PEG CHN Tel: 400-918-9898 Email: sales@sinopeg.com
View More