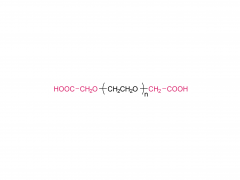
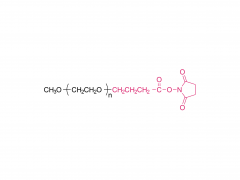
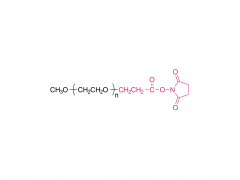
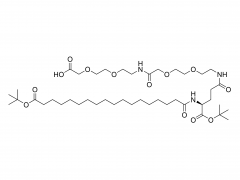
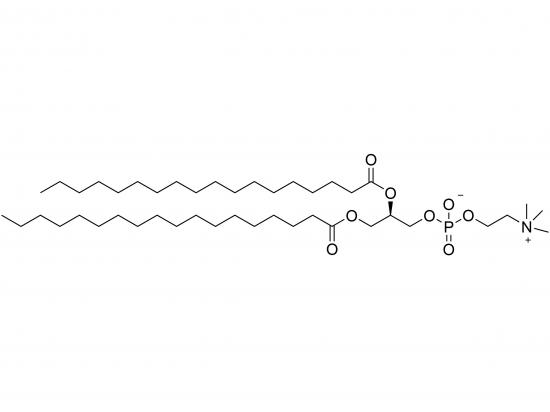
1,2-distearoyl-sn-glycero-3-phosphocholine
Has been registered, DMF 039769, CDE F20230000605
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
Custom synthesis & CMO services are available.
Reference:
1. Deng X, Yang Y, Gan L, et al. Engineering Lipid Nanoparticles to Enhance Intracellular Delivery of Transforming Growth Factor-Beta siRNA (siTGF-β1) via Inhalation for Improving Pulmonary Fibrosis Post-Bleomycin Challenge. Pharmaceutics. 2025;17(2):157. Published 2025 Jan 24. doi:10.3390/pharmaceutics17020157
2. Peng K, Zhao X, Li H, Fu YX, Liang Y. Membrane-IL12 adjuvant mRNA vaccine polarizes pre-effector T cells for optimized tumor control. J Exp Med. 2025;222(9):e20241454. doi:10.1084/jem.20241454
3. Ye T, Zhou J, Guo C, et al. Polyvalent mpox mRNA vaccines elicit robust immune responses and confer potent protection against vaccinia virus. Cell Rep. 2024;43(6):114269. doi:10.1016/j.celrep.2024.114269
4. Wang H, Peng Q, Dai X, et al. A SARS-CoV-2 EG.5 mRNA vaccine induces a broad-spectrum immune response in mice. MedComm (2020). 2025;6(1):e779. Published 2025 Jan 2. doi:10.1002/mco2.779
5. Huang J, Hu Y, Niu Z, et al. Preclinical Efficacy of Cap-Dependent and Independent mRNA Vaccines against Bovine Viral Diarrhea Virus-1. Vet Sci. 2024;11(8):373. Published 2024 Aug 13. doi:10.3390/vetsci11080373
6. Cao H, Zhang X, Cheng J, et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines (Basel). 2025;13(5):497. Published 2025 May 6. doi:10.3390/vaccines13050497
7. Zhang S, Wang X, Zhao T, Yang C, Huang L. Development and Evaluation of the Immunogenic Potential of an Unmodified Nucleoside mRNA Vaccine for Herpes Zoster. Vaccines (Basel). 2025;13(1):68. Published 2025 Jan 13. doi:10.3390/vaccines13010068
8. Wang Z, Tian C, Zhu J, et al. Avian influenza mRNA vaccine encoding hemagglutinin provides complete protection against divergent H5N1 viruses in specific-pathogen-free chickens. J Nanobiotechnology. 2025;23(1):55. Published 2025 Jan 29. doi:10.1186/s12951-025-03156-w
9. Widayat, Wahyu & Hardianto, Ari & Hidayati, Rahma & Neni, Nurainy & Burhanudin, Muhammad & Yusuf, Muhammad & Subroto, Toto. (2025). The Effect of Mixed Lipid Concentrations and Sucrose on the Size of the Lipid Nanoparticles Containing mRNAs. Trends in Sciences. 22. 8985. 10.48048/tis.2025.8985.
10. Li M, Zheng X, Yu X, et al. Potentiating the Efficacy of mRNA Vaccines through NIR-II Imaging-Guided Precise Vaccination. Adv Sci (Weinh). 2025;12(37):e13014. doi:10.1002/advs.202413014
11. Xiao C, Liu X, Liu P, et al. Oncogenic roles of young human de novo genes and their potential as neoantigens in cancer immunotherapy. Cell Genom. 2025;5(9):100928. doi:10.1016/j.xgen.2025.100928
12. Liu D, Wang X, Xu L, et al. Screening lipid nanoparticles using DNA barcoding and qPCR. Colloids Surf B Biointerfaces. 2025;251:114598. doi:10.1016/j.colsurfb.2025.114598
13. Abidin, Muhamad & Yusuf, Muhammad & Widayat, Wahyu & Subroto, Toto & Neni, Nurainy & Hardianto, Ari. (2023). The Implementation of Response Surface Methodology in the Optimization of Lipid Nanoparticle Preparation for Vaccine Development. Trends in Sciences. 21. 7142. 10.48048/tis.2024.7142.
14. Li M, Yi J, Lu Y, et al. Modified PEG-Lipids Enhance the Nasal Mucosal Immune Capacity of Lipid Nanoparticle mRNA Vaccines. Pharmaceutics. 2024;16(11):1423. Published 2024 Nov 7. doi:10.3390/pharmaceutics16111423
15. Hu Z, Pan X, Kang R, et al. Sustained-release hydrogel system for preventing postoperative tumor recurrence through synergistic immune signal enhancement for T cell activation. J Colloid Interface Sci. 2025;699(Pt 1):138158. doi:10.1016/j.jcis.2025.138158
16. Yu W, Chen X, Chen Q, et al. A Safe and Broad-spectrum SARS-CoV-2 mRNA Vaccine with a New Delivery System for In-situ Expression. Virol Sin. Published online September 4, 2025. doi:10.1016/j.virs.2025.09.001
17. Yi J, Lu Y, Liu N, et al. Chitosan and mannose-modified dual-functional mRNA-LNP vaccines for robust systemic and mucosal immune responses. J Control Release. 2025;384:113891. doi:10.1016/j.jconrel.2025.113891
18. Lu Y, Yang Y, Yi J, et al. Design, optimization, and evaluation of lyophilized lipid nanoparticles for mRNA-based pulmonary mucosal vaccination. Mater Today Bio. 2025;32:101813. Published 2025 May 4. doi:10.1016/j.mtbio.2025.101813
19. Wang R, Zhang Y, Du S, Li Y, Ren Y, Lin J. Nanoformulations Downregulating METTL16 Combined with mRNA Tumor Vaccines Suppress Triple-Negative Breast Cancer and Prevent Metastasis. Int J Nanomedicine. 2025;20:8951-8966. Published 2025 Jul 11. doi:10.2147/IJN.S520329
20. Guo T, Wang Y, Chen D, et al. Dual-Drug Loaded Nanobubbles Combined with Sonodynamic and Chemotherapy for Hepatocellular Carcinoma Therapy. Int J Nanomedicine. 2024;19:7367-7381. Published 2024 Jul 19. doi:10.2147/IJN.S460329
21. Li L, Luo M, Zhou L, et al. Glucocorticoid pre-administration improves LNP-mRNA mediated protein replacement and genome editing therapies. Int J Pharm. 2025;672:125282. doi:10.1016/j.ijpharm.2025.125282
22. Yang K, Bai B, Li X, et al. Coordinating interleukin-2 encoding circRNA with immunomodulatory lipid nanoparticles to potentiate cancer immunotherapy. Sci Adv. 2025;11(9):eadn7256. doi:10.1126/sciadv.adn7256
23. Wu K, Xu F, Dai Y, et al. Characterization of mRNA-LNP structural features and mechanisms for enhanced mRNA vaccine immunogenicity. J Control Release. 2024;376:1288-1299. doi:10.1016/j.jconrel.2024.11.007
24. Wan J, Wang Z, Wang L, et al. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. mBio. 2024;15(1):e0177523. doi:10.1128/mbio.01775-23
25. Yu, Juntao & Li, Qian & Luo, Shenggen & Wang, Xiaona & Cheng, Qiang & hu, Rongkuan. (2024). Targeted LNPs deliver mRNA encoding IL-15 superagonists to balance efficacy and toxicity in cancer therapy. 10.1101/2024.01.11.575299.
26. Sun, Zhen & Liu, Yuxiao & Zhang, Haoyi & Ge, Ting & Pan, Yuting & Liu, Yang & Wu, Miaomiao & Shan, Tao & Wu, Qi & Zhu, Guoqiang & Chen, Kangming. (2025). Next-Generation saRNA Platforms: Systematic Screening and Engineering Enhances Superior Protein Expression and Organ-Specific Targeting for RNA Therapeutics. 10.1101/2025.03.30.644708.
27. Luo C, Li Y, Liu H, et al. Intracellular trafficking of lipid nanoparticles is hindered by cholesterol. Int J Pharm. 2025;671:125240. doi:10.1016/j.ijpharm.2025.125240
28. Liu X, Li Z, Li X, et al. A single-dose circular RNA vaccine prevents Zika virus infection without enhancing dengue severity in mice. Nat Commun. 2024;15(1):8932. Published 2024 Oct 16. doi:10.1038/s41467-024-53242-0
29. Wang L, Wan J, He W, et al. IL-7 promotes mRNA vaccine-induced long-term immunity. J Nanobiotechnology. 2024;22(1):716. Published 2024 Nov 16. doi:10.1186/s12951-024-02993-5
30. Yufeng Zhang,Liuwei Zhang,Hui Gao, et al. Multi-functional lipid nanoformulations for enhancing the efficacy of mRNA tumor vaccines by reversing tumor immunosuppressive microenvironment[J].Nano Today, 2025, 63.DOI:10.1016/j.nantod.2025.102757.
31. Shao X, Liu L, Weng C, et al. Leveraging an mRNA Platform for the Development of Vaccines Against Egg Allergy. Vaccines (l). 2025;13(5):448. Published 2025 Apr 24. doi:10.3390/vaccines13050448