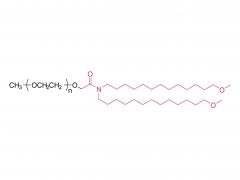
((4-hydroxybutyl) azanediyl) bis (hexane-6,1-diyl) bis (2-hexyldecanoate)
Has been registered, DMF 038107, CDE F20220000045
Custom synthesis & CMO services are available.
License is required from license holder.
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
Reference:
1. Deng X, Yang Y, Gan L, et al. Engineering Lipid Nanoparticles to Enhance Intracellular Delivery of Transforming Growth Factor-Beta siRNA (siTGF-β1) via Inhalation for Improving Pulmonary Fibrosis Post-Bleomycin Challenge. Pharmaceutics. 2025;17(2):157. Published 2025 Jan 24. doi:10.3390/pharmaceutics17020157
2. Nguyen CM, Vu TT, Nguyen MN, et al. Neoantigen-based mRNA vaccine exhibits superior anti-tumor activity compared to synthetic long peptides in an in vivo lung carcinoma model. Cancer Immunol Immunother. 2025;74(4):145. Published 2025 Mar 12. doi:10.1007/s00262-025-03992-7
3. Cheng X, Zheng X, Tao K, et al. Freezing induced incorporation of betaine in lipid nanoparticles enhances mRNA delivery. Nat Commun. 2025;16(1):4700. Published 2025 May 20. doi:10.1038/s41467-025-60040-9
4. Liu S, Wen Y, Shan X, et al. Charge-assisted stabilization of lipid nanoparticles enables inhaled mRNA delivery for mucosal vaccination. Nat Commun. 2024;15(1):9471. Published 2024 Nov 2. doi:10.1038/s41467-024-53914-x
5. Kozlova A, Pateev I, Shepelkova G, et al. A Cap-Optimized mRNA Encoding Multiepitope Antigen ESAT6 Induces Robust Cellular and Humoral Immune Responses Against Mycobacterium tuberculosis. Vaccines (Basel). 2024;12(11):1267. Published 2024 Nov 9. doi:10.3390/vaccines12111267
6. Jiang L, Zhou W, Liu F, et al. An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models. Vaccines (Basel). 2025;13(3):327. Published 2025 Mar 19. doi:10.3390/vaccines13030327
7. Li M, Zheng X, Yu X, et al. Potentiating the Efficacy of mRNA Vaccines through NIR-II Imaging-Guided Precise Vaccination. Adv Sci (Weinh). 2025;12(37):e13014. doi:10.1002/advs.202413014
8. Kim S, Jeon JH, Kim M, et al. Innate immune responses against mRNA vaccine promote cellular immunity through IFN-β at the injection site. Nat Commun. 2024;15(1):7226. Published 2024 Aug 27. doi:10.1038/s41467-024-51411-9
9. Liu D, Wang X, Xu L, et al. Screening lipid nanoparticles using DNA barcoding and qPCR. Colloids Surf B Biointerfaces. 2025;251:114598. doi:10.1016/j.colsurfb.2025.114598
10. Ao D, Peng D, He C, et al. A promising mRNA vaccine derived from the JN.1 spike protein confers protective immunity against multiple emerged Omicron variants. Mol Biomed. 2025;6(1):13. Published 2025 Mar 4. doi:10.1186/s43556-025-00258-7
11. Xu L, Chen R, Wang X, Liu D, Liu Y, Zhao CX. DNA Barcoding-Enabled Tracking of Lipid Nanoparticles: Drug-Loading-Dependent Biodistribution and Tumor Microenvironment Targeting. Adv Healthc Mater. 2025;14(24):e2501914. doi:10.1002/adhm.202501914
12. Hassanel DNBP, Pilkington EH, Ju Y, Kent SJ, Pouton CW, Truong NP. Replacing poly(ethylene glycol) with RAFT lipopolymers in mRNA lipid nanoparticle systems for effective gene delivery. Int J Pharm. 2024;665:124695. doi:10.1016/j.ijpharm.2024.124695
13. Hassanel DNBP, Ju Y, Takanashi A, et al. Influence of hydrophilic polymers on the accelerated blood clearance of mRNA lipid nanoparticles upon repeated administration. Nanoscale Horiz. Published online October 1, 2025. doi:10.1039/d5nh00230c
14. Li L, Luo M, Zhou L, et al. Glucocorticoid pre-administration improves LNP-mRNA mediated protein replacement and genome editing therapies. Int J Pharm. 2025;672:125282. doi:10.1016/j.ijpharm.2025.125282
15. Ohta N, Matsuzaki T, Nakai M, Tabata Y, Nimura K. Combining mRNA with PBS and calcium ions improves the efficiency of the transfection of mRNA into tumors. Mol Ther Nucleic Acids. 2024;35(3):102273. Published 2024 Jul 17. doi:10.1016/j.omtn.2024.102273
16. Yang K, Bai B, Li X, et al. Coordinating interleukin-2 encoding circRNA with immunomodulatory lipid nanoparticles to potentiate cancer immunotherapy. Sci Adv. 2025;11(9):eadn7256. doi:10.1126/sciadv.adn7256
17. Meng Y, Ba Q, Yao J, et al. Targeted Reprogramming of Tumor Cells by Digoxin-Loaded Immunogenic Nanoparticles Enhances Immunity Against Disseminated Tumor Cells. Adv Healthc Mater. Published online September 4, 2025. doi:10.1002/adhm.202502881
18. Ren L, Zhao Z, Chao Y, et al. Optimization of Lipid Nanoparticles with Robust Efficiency for the Delivery of Protein Therapeutics to Augment Cancer Immunotherapy. Adv Sci (Weinh). 2025;12(17):e2500844. doi:10.1002/advs.202500844
19. Wang Y, Luo G, Wang H, et al. Evaluating cell cycle- and autophagy-associated cellular accumulation of lipid-based nanoparticles. Nat Commun. 2025;16(1):5964. Published 2025 Jul 1. doi:10.1038/s41467-025-60962-4
20. Sui, Meihua & Wang, Yisha & Luo, Gan & Wang, Haiyang & Zheng, Yue & Zhou, Wenbin & Lin, Junrong & Chen, Baocheng. (2024). Construction and Application of a Technical Platform for Determining Cell Cycle- and Autophagy-Associated Cellular Uptake of Lipid-Based Nanoparticles. 10.21203/rs.3.rs-3974581/v1.
21. Wang H, Wang Y, Yuan C, et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines. 2023;8(1):169. Published 2023 Nov 2. doi:10.1038/s41541-023-00766-z