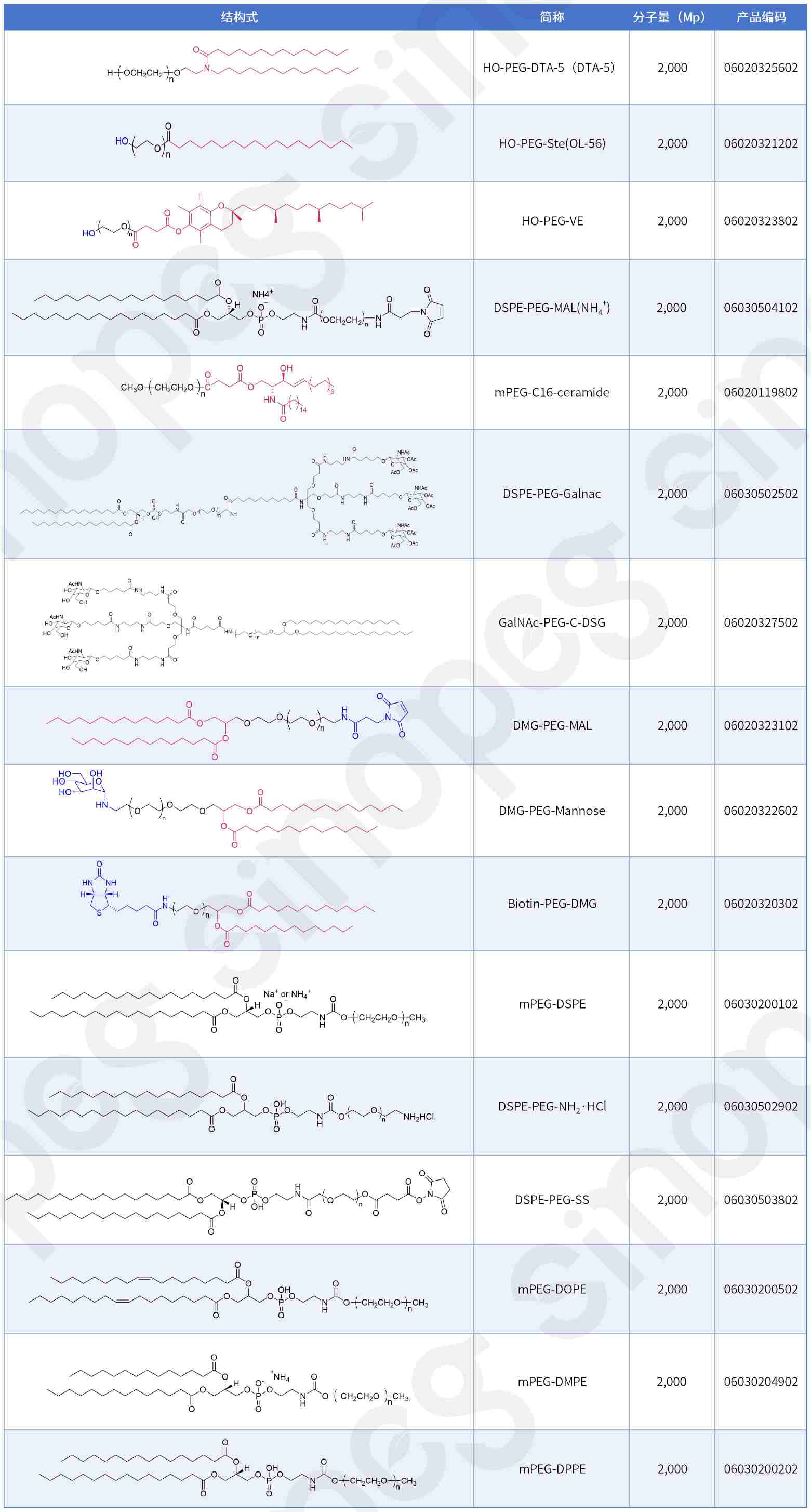
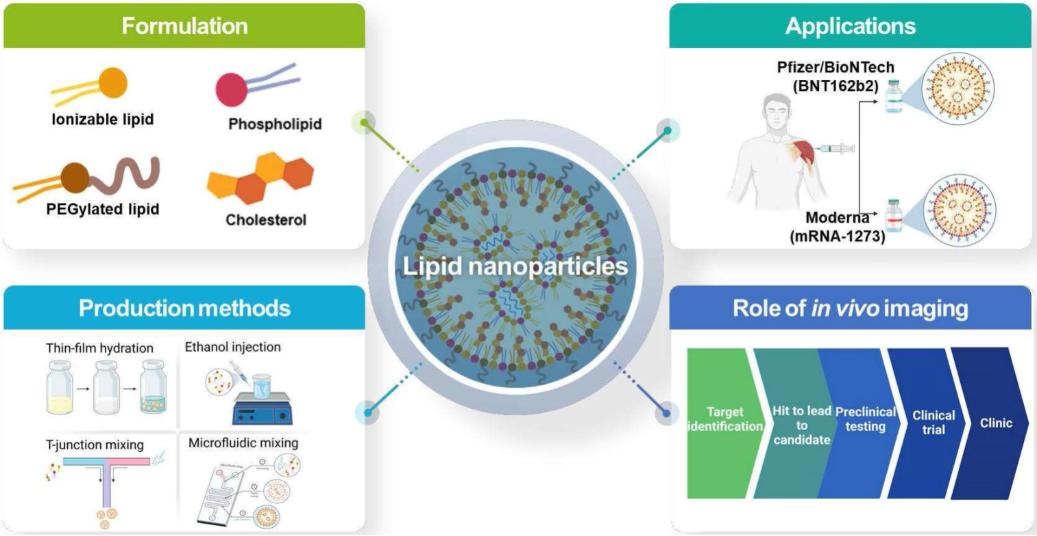
Lipid Nanoparticles (LNPs), as one of the most successful nano-delivery carriers today, demonstrate exceptional capabilities in delivering cytotoxic chemotherapeutic drugs, antibiotics, and nucleic acid therapeutics. The structural choice of PEGylated lipids, a core component of LNPs, decisively influences the formulation's safety, stability, and immunogenicity.
Have you ever been troubled by immune responses triggered by PEG in LNP formulations? Pre-existing anti-PEG antibodies have become an unavoidable challenge in the clinical application of mRNA drugs. Today, PEG lipids with hydroxyl terminals (HO-PEG) are emerging as the "invisibility cloak" for the new generation of LNPs—significantly reducing immune recognition and enhancing delivery efficiency. SINOPEG brings you HO-PEG lipids with independent intellectual property rights to address pre-existing anti-PEG antibodies.
SINOPEG: The Leader in Domestic HO-PEG Lipids
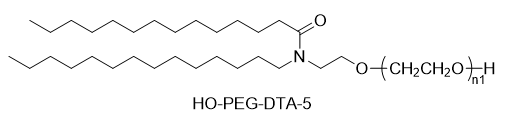
SINOPEG has always been committed to the research and development of new technologies and products. Recently, various new lipid structures are available off-the-shelf, especially the independently developed DTA-5 (Patent No.: ZL202280003648.7), a PEG lipid containing a hydroxyl terminal. HO-PEG-DTA-5 has established a comprehensive global intellectual property portfolio, covering major markets including China, the United States, and Europe. Furthermore, this lipid is nearing completion of drug master file (DMF) submissions in both China and the US. Its structure is as follows:
HO-PEG Lipids: Why Are They the Preferred Choice for Next-Generation LNPs?
In recent years, PEG lipids with hydroxyl terminals (HO-PEG) have gradually become a focus in the development of next-generation LNPs due to their significant advantages in reducing immunogenicity and avoiding recognition by pre-existing anti-PEG antibodies. Moderna, a global leader in mRNA therapeutics, extensively uses such lipids in its numerous preclinical and clinical formulations, further validating their potential.
Not long ago, Moderna published an important study in Nature Communications titled "Characterizing the mechanism of action for mRNA therapeutics for the treatment of propionic acidemia, methylmalonic acidemia, and phenylketonuria." This paper systematically elucidates the mechanisms of action of three mRNA therapies for treating metabolic diseases (including Propionic Acidemia (PA), Methylmalonic Acidemia (MMA), and Phenylketonuria (PKU)): mRNA-3927 (investigational treatment for PA), mRNA-3705 (investigational treatment for MMA), and mRNA-3210 (investigational treatment for PKU). All three mRNA therapies utilize an LNP delivery system and demonstrated favorable pharmacokinetic/pharmacodynamic (PK/PD) responses in mouse models, including target mRNA expression, increased protein activity, and decreased levels of relevant metabolites.
These three therapies in the study employed a unified LNP formulation: the cationic lipid was SM-86, and the PEG lipid was OL-56.
SM-86 has been mentioned in previous non-clinical review reports for SPIKEVAX®. It is a lipid structurally similar to SM-102, and in intravenous injection studies in rats, its pharmacokinetics, tissue distribution, and excretion profiles were also similar to SM-102. It corresponds to Lipid 5 mentioned in Moderna's earlier research on lipid delivery carriers.
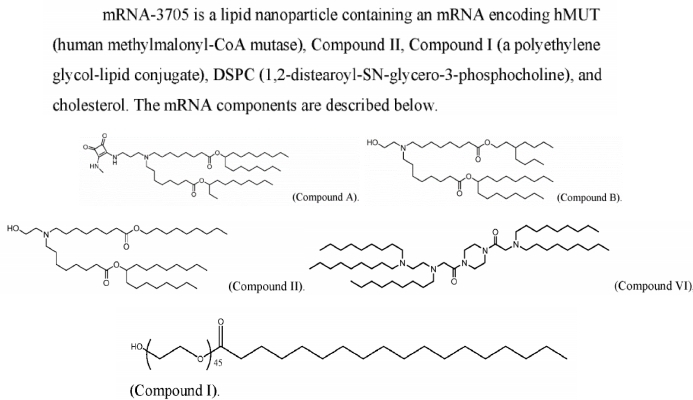
OL-56, based on previously disclosed patent information from Moderna, is described as a polyethylene glycolated fatty acid derivative (belonging to the Formula (VI) structure), with its distinguishing feature being a hydroxyl terminal (-OH) on the PEG, i.e., an HO-PEG lipid. This type of PEG lipid has been used in conjunction with various ionizable lipids (such as Compound A, B, II, VI, etc.) across multiple patent documents, demonstrating good compatibility and delivery efficiency. Patent WO2022246020A1 discloses the formulation for mRNA-3705, confirming that the Compound I disclosed in the patent is the OL-56 mentioned in the article.
Based on pharmacokinetic (PK) and pharmacodynamic (PD) data from preclinical mouse models, the research team developed preclinical PK/PD models for each mRNA therapeutic. These models were then successfully applied to human dose prediction using allometric scaling of model parameters. Specifically, for the Propionic Acidemia (PA) and Methylmalonic Acidemia (MMA) studies, data from mice, rats, and monkeys were integrated, and interspecies scaling methods were used to predict the First-In-Human (FIH) dose. For Phenylketonuria (PKU), predictions were primarily based on a disease-relevant model – the PAHenu2 mouse model.
As shown in Table 1 below, based on the PK/PD modeling results, the estimated FIH doses for the three mRNA therapeutics (mRNA-3927, mRNA-3705, and mRNA-3210) are 0.3 mg/kg, 0.1 mg/kg, and 0.4 mg/kg, respectively. These values are significantly lower than their corresponding No Observed Adverse Effect Levels (NOAELs): PA 3 mg/kg, MMA 5 mg/kg, and PKU 3 mg/kg, achieving safety margins of 10, 50, and 7.5 respectively, indicating excellent potential for clinical safety.
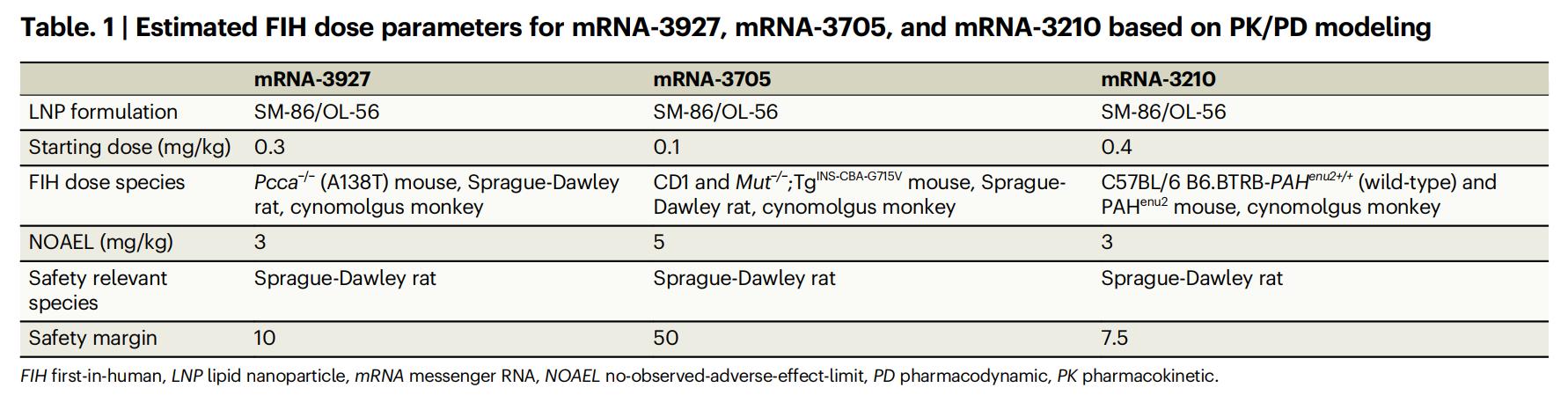
Table 1: Estimated FIH Dose Parameters for mRNA-3927, mRNA-3705, and mRNA-3210 Based on PK/PD Modeling
Recently, a research team led by Professor Zhan Changyou from the School of Basic Medical Sciences at Fudan University also published a related study on PEG lipids with OH terminals in the preprint journal bioRxiv. This study systematically explored the breakthrough potential of PEG lipids with hydroxyl terminals (OH-terminal) in evading recognition by pre-existing anti-PEG antibodies in humans. Through systematic screening of large-sample, multi-center clinical sera, the research found that the recognition of PEG materials by widely prevalent anti-polyethylene glycol (anti-PEG) antibodies in the population exhibits significant terminal selectivity. Among these, hydroxyl-PEG (HO-PEG) demonstrated exceptional "immune escape" capability – significantly avoiding binding with pre-existing anti-PEG antibodies.
The study comprehensively used ELISA, Isothermal Titration Calorimetry (ITC), and various in vitro functional assays to systematically compare the binding characteristics of PEG materials with different terminal structures (including MeO-, HO-, NH₂-, and COOH-PEG) to antibodies. The results in the figure below show that HO-PEG exhibited the lowest immunorecognition rates across all tests, with positive binding signals reduced by over 90%. Particularly noteworthy, cross-species comparisons revealed that human pre-existing antibodies primarily recognize the PEG terminal structure, whereas antibodies induced in animal models often target the PEG repeating units. This key difference suggests that the advantages of HO-PEG are highly specific and hold significant translational potential in clinical settings.
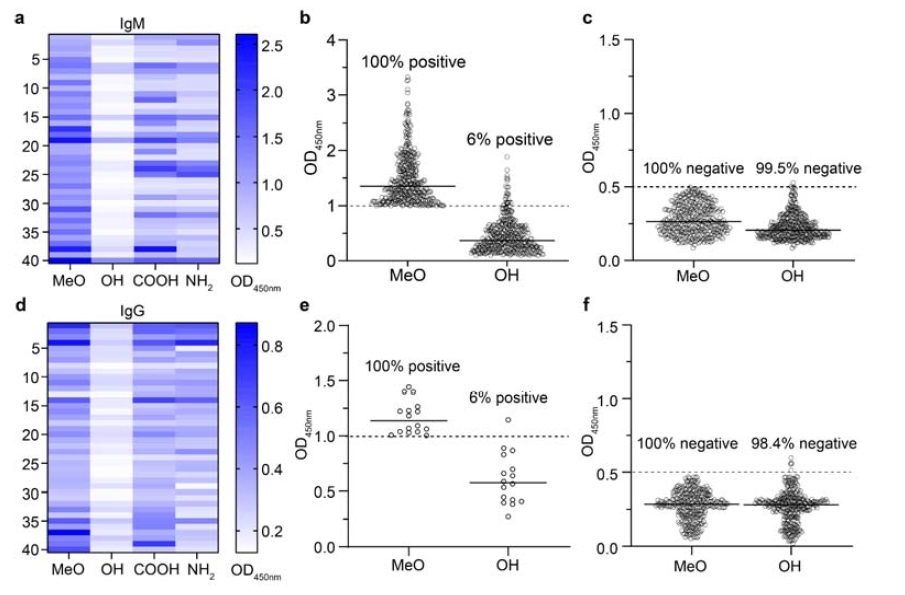
Figure: Terminal Selectivity of Human Pre-existing Anti-PEG Antibodies
In summary, this study demonstrated that compared to traditional methoxy-PEG (MeO-PEG), lipid nanoparticles (LNPs) modified with HO-PEG can substantially reduce complement activation levels, decrease anaphylatoxin generation, significantly enhance the plasma stability of LNPs, and reduce non-target macrophage uptake in antibody-positive human serum. These improvements provide a practical new material strategy for enhancing the delivery efficiency and in vivo performance of LNP-based drugs while reducing clinical immune-related side effects.
Both the Moderna and Fudan University studies corroborate the significant value of PEG lipids with hydroxyl terminals as key excipients in improving formulation safety and reducing immune responses. The adoption of such advanced functional lipid materials is becoming a crucial direction in the development of next-generation nucleic acid drug delivery systems.
SINOPEG not only provides HO-PEG lipids but also offers other novel PEG-lipid structures: DSPE-PEG-MAL, GaINAC-PEG-C-DSG, OL-56 (HO-PEG-Ste), mPEG-C16-ceramide, mPEG-DHE-, Biotin-PEG-DMG, DMG-PEG-MAL, HO-PEG-VE, DMG-PEG-Mannose, DSPE-PEG-Galnac, mPEG-DPPE(14:0 PEG2000), mPEG-DMPE(16:0 PEG2000 PE-2K), mPEG-DOPE(18:1 PEG2000 PE-2K).