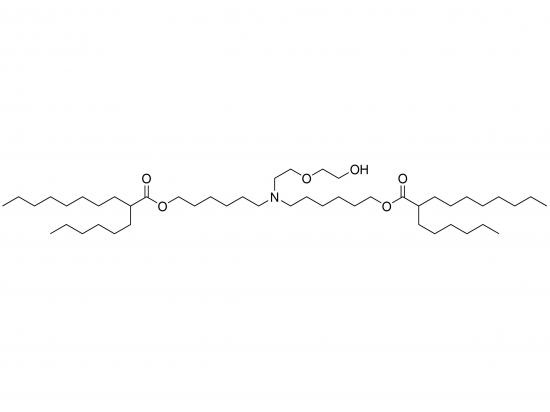
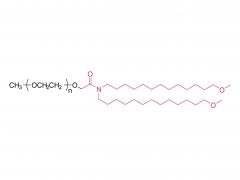
Has been registered, DMF 039452, CDE F20230000445
Own patented product, patent No. 202110839008.7
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
Reference:
1. Deng X, Yang Y, Gan L, et al. Engineering Lipid Nanoparticles to Enhance Intracellular Delivery of Transforming Growth Factor-Beta siRNA (siTGF-β1) via Inhalation for Improving Pulmonary Fibrosis Post-Bleomycin Challenge. Pharmaceutics. 2025;17(2):157. Published 2025 Jan 24. doi:10.3390/pharmaceutics17020157
2. Huang J, Hu Y, Niu Z, et al. Preclinical Efficacy of Cap-Dependent and Independent mRNA Vaccines against Bovine Viral Diarrhea Virus-1. Vet Sci. 2024;11(8):373. Published 2024 Aug 13. doi:10.3390/vetsci11080373