|
Number |
Mn |
|
06020310802 |
2,000 |
|
06020310806 |
5,000 |
|
06020310809 |
10,000 |
|
06020310812 |
20,000 |
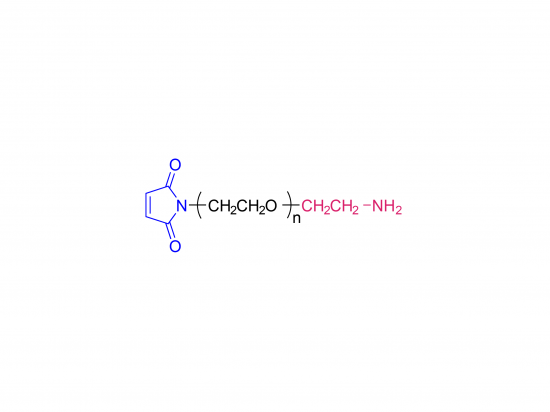
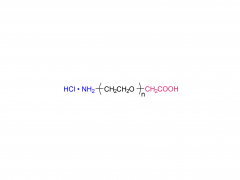
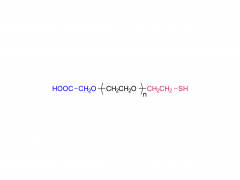
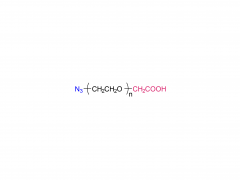
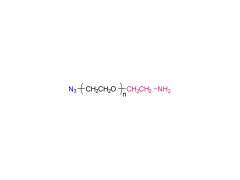
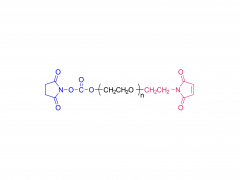
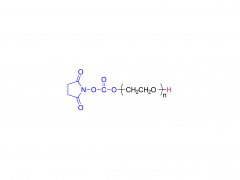
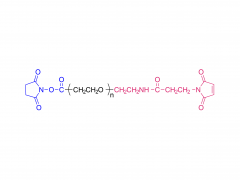
α-Maleimidyl-ω-amino poly(ethylene glycol) is available with molecular weight 2K, 5K, 10K and 20KDa, other M.W. may be available by custom synthesis. The packaging sizes are available for 1g, 10g and 100g. Please contact us for bulk and GMP grade PEGs pricing and packaging size. MW 2000 Da, MW 5000 Da, MW 10000 Da, MW 20000 Da, MW 40000 Da are available.
Xiamen Sinopeg Biotech Co., Ltd. is dedicated to drug delivery systems and related medical device business, focusing on the research, development, production and sales of high-end drug delivery carriers/auxiliary materials/APIs, medical materials, including but not limited to polyethylene glycol derivatives, lipid products, blood sugar control drug modifiers, block copolymers, ADC/ProTAC linkers, biodegradable polymers, exosomes, viral-like particles, as well as providing CDMO and solution services. These products are widely used in long-acting protein/peptide drugs, mRNA vaccines, small nucleic acid drugs, blood sugar control drugs, macromolecular micelle drugs, liposome drugs, gene therapy drugs, immunosuppressants, ADC drugs, ProTAC drugs, medical hydrogels, and other fields, placing the company in a leading position in the industry.
SINOPEG holds 40+ patents, with 80+ more pending, and 30+ products filed in DMF/CDE. The company has ISO 9001/ISO 13485/ISO 14001/ISO 45001 certification. The laboratory and production workshop are designed and built in accordance with the cGMP standard of FDA. We follow the requirements of ICH-Q7A to organize production at scale, to provide high quality drug delivery products and services to customers globally.
Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
1. Galindo-Camacho, R. M., et al., Cell penetrating peptides-functionalized Licochalcone-A-loaded PLGA nanoparticles for ocular inflammatory diseases: Evaluation of in vitro anti-proliferative effects, stabilization by freeze-drying and characterization of an in-situ forming gel, International Journal of Pharmaceutics, 2023, V. 639.
2. Sanna, V., et al., Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition, European Journal of Medicinal Chemistry, 2022,114121.
3. Distasio, N, et al., VCAM‐1‐Targeted Gene Delivery Nanoparticles Localize to Inflamed Endothelial Cells and Atherosclerotic Plaques. Advanced Therapeutics. 2021, 4(2):2000196.
4. Xia, C., et al., Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase A silencing for enhanced cancer immunochemotherapy, Journal of Controlled Release, 2021, V. 335, P. 557-574.
5. Li, H., et al., Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma, International Journal of Biological Macromolecules, 2018, V. 107A, P. 204-211.
6. Jiang, Y., et al., TEMPO-oxidized starch nanoassemblies of negligible toxicity compared with polyacrylic acids for high performance anti-cancer therapy, International Journal of Pharmaceutics, 2018, V. 547 (1–2), p. 520-529.7. Chen, G., et al., NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier, Journal of Controlled Release, 2018, V. 282, P. 148-155.