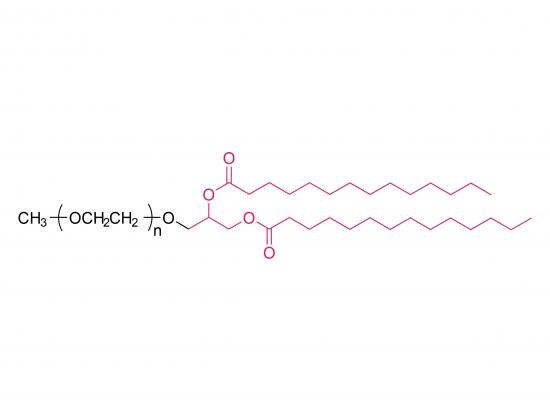
Methoxypoly(ethylene glycol) dimyristoyl glycerol
Has been registered, DMF 038025, CDE F20210000399
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.
Custom synthesis & CMO services are available.
License is required from license holder.
Reference:
1. Deng X, Yang Y, Gan L, et al. Engineering Lipid Nanoparticles to Enhance Intracellular Delivery of Transforming Growth Factor-Beta siRNA (siTGF-β1) via Inhalation for Improving Pulmonary Fibrosis Post-Bleomycin Challenge. Pharmaceutics. 2025;17(2):157. Published 2025 Jan 24. doi:10.3390/pharmaceutics17020157
2. Peng K, Zhao X, Li H, Fu YX, Liang Y. Membrane-IL12 adjuvant mRNA vaccine polarizes pre-effector T cells for optimized tumor control. J Exp Med. 2025;222(9):e20241454. doi:10.1084/jem.20241454
3. Zhu C, Roa N, Neathery E et al. Comparative technical and operational assessment of current and emerging bench-scale lipid nanoparticle platforms for production of mRNA vaccines [version 1]. VeriXiv 2025, 2:96 (https://doi.org/10.12688/verixiv.982.1)
4. Ye T, Zhou J, Guo C, et al. Polyvalent mpox mRNA vaccines elicit robust immune responses and confer potent protection against vaccinia virus. Cell Rep. 2024;43(6):114269. doi:10.1016/j.celrep.2024.114269
5. Wang H, Peng Q, Dai X, et al. A SARS-CoV-2 EG.5 mRNA vaccine induces a broad-spectrum immune response in mice. MedComm (2020). 2025;6(1):e779. Published 2025 Jan 2. doi:10.1002/mco2.779
6. Huang J, Hu Y, Niu Z, et al. Preclinical Efficacy of Cap-Dependent and Independent mRNA Vaccines against Bovine Viral Diarrhea Virus-1. Vet Sci. 2024;11(8):373. Published 2024 Aug 13. doi:10.3390/vetsci11080373
7. Cao H, Zhang X, Cheng J, et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines (Basel). 2025;13(5):497. Published 2025 May 6. doi:10.3390/vaccines13050497
8. Zhang S, Wang X, Zhao T, Yang C, Huang L. Development and Evaluation of the Immunogenic Potential of an Unmodified Nucleoside mRNA Vaccine for Herpes Zoster. Vaccines (Basel). 2025;13(1):68. Published 2025 Jan 13. doi:10.3390/vaccines13010068
9. Wang Z, Tian C, Zhu J, et al. Avian influenza mRNA vaccine encoding hemagglutinin provides complete protection against divergent H5N1 viruses in specific-pathogen-free chickens. J Nanobiotechnology. 2025;23(1):55. Published 2025 Jan 29. doi:10.1186/s12951-025-03156-w
10. Widayat, Wahyu & Hardianto, Ari & Hidayati, Rahma & Neni, Nurainy & Burhanudin, Muhammad & Yusuf, Muhammad & Subroto, Toto. (2025). The Effect of Mixed Lipid Concentrations and Sucrose on the Size of the Lipid Nanoparticles Containing mRNAs. Trends in Sciences. 22. 8985. 10.48048/tis.2025.8985.
11. Xiao C, Liu X, Liu P, et al. Oncogenic roles of young human de novo genes and their potential as neoantigens in cancer immunotherapy. Cell Genom. 2025;5(9):100928. doi:10.1016/j.xgen.2025.100928
12. Liu D, Wang X, Xu L, et al. Screening lipid nanoparticles using DNA barcoding and qPCR. Colloids Surf B Biointerfaces. 2025;251:114598. doi:10.1016/j.colsurfb.2025.114598
13. Wang Z, Chen Y, Wu H, et al. Intravenous administration of IL-12 encoding self-replicating RNA-lipid nanoparticle complex leads to safe and effective antitumor responses. Sci Rep. 2024;14(1):7366. Published 2024 Mar 28. doi:10.1038/s41598-024-57997-w
14. Yu W, Chen X, Chen Q, et al. A Safe and Broad-spectrum SARS-CoV-2 mRNA Vaccine with a New Delivery System for In-situ Expression. Virol Sin. Published online September 4, 2025. doi:10.1016/j.virs.2025.09.001
15. Shi R, Liu X, Wang Y, et al. Long-term stability and immunogenicity of lipid nanoparticle COVID-19 mRNA vaccine is affected by particle size. Hum Vaccin Immunother. 2024;20(1):2342592. doi:10.1080/21645515.2024.2342592
16. Lv K, Yu Z, Wang J, et al. Discovery of Ketal-Ester Ionizable Lipid Nanoparticle with Reduced Hepatotoxicity, Enhanced Spleen Tropism for mRNA Vaccine Delivery. Adv Sci (Weinh). 2024;11(45):e2404684. doi:10.1002/advs.202404684
17. Wang R, Zhang Y, Du S, Li Y, Ren Y, Lin J. Nanoformulations Downregulating METTL16 Combined with mRNA Tumor Vaccines Suppress Triple-Negative Breast Cancer and Prevent Metastasis. Int J Nanomedicine. 2025;20:8951-8966. Published 2025 Jul 11. doi:10.2147/IJN.S520329
18. Li L, Luo M, Zhou L, et al. Glucocorticoid pre-administration improves LNP-mRNA mediated protein replacement and genome editing therapies. Int J Pharm. 2025;672:125282. doi:10.1016/j.ijpharm.2025.125282
19. Ohta N, Matsuzaki T, Nakai M, Tabata Y, Nimura K. Combining mRNA with PBS and calcium ions improves the efficiency of the transfection of mRNA into tumors. Mol Ther Nucleic Acids. 2024;35(3):102273. Published 2024 Jul 17. doi:10.1016/j.omtn.2024.102273
20. Wu K, Xu F, Dai Y, et al. Characterization of mRNA-LNP structural features and mechanisms for enhanced mRNA vaccine immunogenicity. J Control Release. 2024;376:1288-1299. doi:10.1016/j.jconrel.2024.11.007
21. Wan J, Wang Z, Wang L, et al. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. mBio. 2024;15(1):e0177523. doi:10.1128/mbio.01775-23
22. Yu, Juntao & Li, Qian & Luo, Shenggen & Wang, Xiaona & Cheng, Qiang & hu, Rongkuan. (2024). Targeted LNPs deliver mRNA encoding IL-15 superagonists to balance efficacy and toxicity in cancer therapy. 10.1101/2024.01.11.575299.
23. Sun, Zhen & Liu, Yuxiao & Zhang, Haoyi & Ge, Ting & Pan, Yuting & Liu, Yang & Wu, Miaomiao & Shan, Tao & Wu, Qi & Zhu, Guoqiang & Chen, Kangming. (2025). Next-Generation saRNA Platforms: Systematic Screening and Engineering Enhances Superior Protein Expression and Organ-Specific Targeting for RNA Therapeutics. 10.1101/2025.03.30.644708.
24. Luo C, Li Y, Liu H, et al. Intracellular trafficking of lipid nanoparticles is hindered by cholesterol. Int J Pharm. 2025;671:125240. doi:10.1016/j.ijpharm.2025.125240
25. Liu X, Li Z, Li X, et al. A single-dose circular RNA vaccine prevents Zika virus infection without enhancing dengue severity in mice. Nat Commun. 2024;15(1):8932. Published 2024 Oct 16. doi:10.1038/s41467-024-53242-0
26. Chen YC, Lee YL, Lee CA, Lin TY, Hwu EE, Cheng PC. Development of a Lipid-encapsulated TGFβRI-siRNA Drug for Liver Fibrosis Induced by Schistosoma mansoni. PLoS Negl Trop Dis. 2024;18(9):e0012502. Published 2024 Sep 12. doi:10.1371/journal.pntd.0012502
27. Wang L, Wan J, He W, et al. IL-7 promotes mRNA vaccine-induced long-term immunity. J Nanobiotechnology. 2024;22(1):716. Published 2024 Nov 16. doi:10.1186/s12951-024-02993-5
28. Bian X, Guo Q, Yau LF, et al. Berberine-inspired ionizable lipid for self-structure stabilization and brain targeting delivery of nucleic acid therapeutics. Nat Commun. 2025;16(1):2368. Published 2025 Mar 10. doi:10.1038/s41467-025-57488-0
29. Yufeng Zhang,Liuwei Zhang,Hui Gao,等.Multi-functional lipid nanoformulations for enhancing the efficacy of mRNA tumor vaccines by reversing tumor immunosuppressive microenvironment[J].Nano Today, 2025, 63.DOI:10.1016/j.nantod.2025.102757.
30. Shao X, Liu L, Weng C, et al. Leveraging an mRNA Platform for the Development of Vaccines Against Egg Allergy. Vaccines (l). 2025;13(5):448. Published 2025 Apr 24. doi:10.3390/vaccines13050448