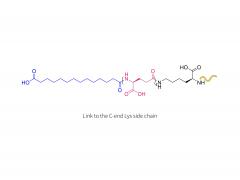
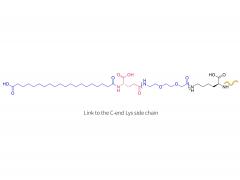
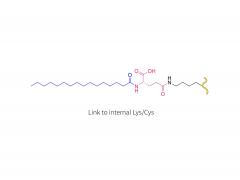
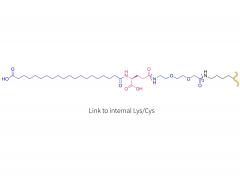
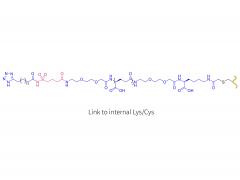
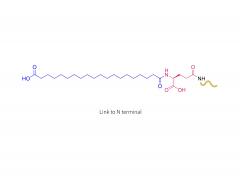
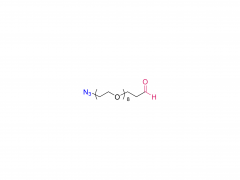
License is required from license holder.
SINOPEG is serving pharmaceutical and medical device companies around the globe, with product presence in various pharmaceutical/device development pipeline (pre-clinical, clinical, and post authorization large scale supply). Our facility is ISO9001 and ISO13485 certified, and is operating according to ICH Q7A guidelines to produce products for pharmaceutical companies.
Please contact us at sales@sinopeg.com for PEG derivatives. Our online catalog or inventory may not listed or have all molecular weights and functional groups, which may be available by custom synthesis. Please contact us at sales@sinopeg.com for quotation and availability.