Published: 16 May 2018; DOI:10.1007/s40242-018-8023-3
Chemical Research in Chinese Universities volume 34, pages428–433 (2018)
Site-specific PEGylation of Human Growth Hormone by Mutated Sortase A
Hui Shi, Qingyang Shi, James T. Oswald, Ying Gao, Leijiao Li & Yunhui Li
Abstract
Human growth hormone(hGH), a classic therapeutic protein, which promotes growth and wound healing, is released from the pituitary gland. As a protein drug, its short half-life is its main barrier to therapeutic efficacy. Various strategies have been designed to prolong its serum half-life, the most common of which is the conjugation with polyethylene glycol(PEG), as this has been shown to significantly extend protein’s serum half-life. However, PEGylation often results in random conjugation, which can lead to impaired protein function and hinder purification, characterization and evaluation of the PEGylated protein. Therefore, site specific PEGylation is a promising direction for PEG-protein conjugation. Here we took advantages of the mutated sortase A(7M) enzyme, which can enzymatically ligate the universal α-amino acids to a C-terminal tagged protein. This then allows specific modification of the C-terminal of hGH with PEG. This site-specific bound PEG-hGH has similar efficacy, receptor binding and cell proliferation as wild-type hGH; however, pharmacokinetic analysis demonstrates that its serum half-life is almost 24 times that of wild-type hGH. Herein, we provided a promising advancement in the development of site specific PEGylated therapeutic proteins.
Related products
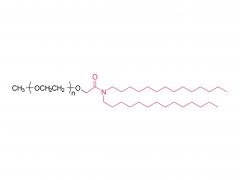
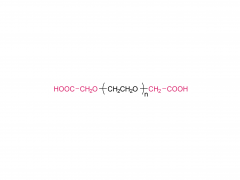
Abbreviation: mPEG-NH2
Name: Methoxypoly(ethylene glycol) amine
For more product information, please contact us at:
US Tel: 1-844-782-5734
US Tel: 1-844-QUAL-PEG
CHN Tel: 400-918-9898
Email: sales@sinopeg.com