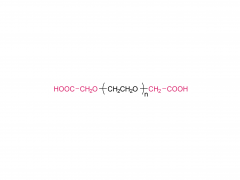
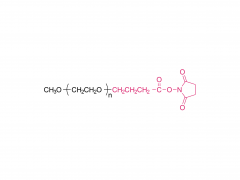
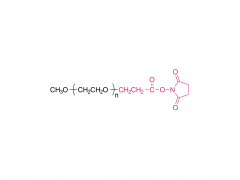
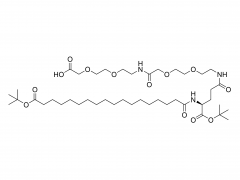
Br J Haematol. 2020 May;189(3):442-451. doi: 10.1111/bjh.16254. Epub 2019 Dec 27. Acute lymphoblastic leukaemia patients treated with PEGasparaginase develop antibodies to PEG and the succinate linker Abstract Polyethylene glycol (PEG) conjugated asparaginase (PEGasparaginase) is essential for treatment of paediatric acute lymphoblastic leukaemia. We developed an assay identifying antibodies against the PEG-moiety, the linker and the drug itself in patients experiencing hypersensitivity reactions to PEGasparaginase. Eighteen patients treated according to the DCOG ALL-11 protocol, with a neutralizing hypersensitivity reaction to PEGasparaginase to the first PEGasparaginase doses in induction (12 patients) or during intensification after interruption of several months (6 patients) were included. ELISA was used to measure antibodies, coating with the succinimidyl succinate linker conjugated to BSA, PEGfilgrastim and Escherichia coli asparaginase, and using hydrolysed PEGasparaginase and mPEG5,000 for competition. Anti-PEG antibodies were detected in all patients (IgG 100%; IgM 67%) of whom 39% had anti-PEG antibodies exclusively. Pre-existing anti-PEG antibodies were also detected in patients who not previously received a PEGylated therapeutic (58% IgG; 21% IgM). Antibodies against the SS-linker were predominantly detected during induction (50% IgG; 42% IgM). Anti-asparaginase antibodies were detected in only 11% during induction but 94% during intensification. In conclusion, anti-PEG and anti-SS-linker antibodies predominantly play a role in the immunogenic response to PEGasparaginase during induction. Thus, switching to native E. coli asparaginase would be an option for adequate asparaginase treatment. Keywords: PEGasparaginase; acute lymphoblastic leukemia; antibodies.
View More