Riding the Waves, Connecting Globally | Dual Exhibitions, SINOPEG Shines in Europe!
November 20,2025.
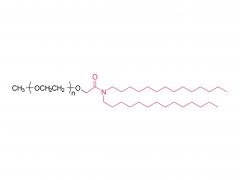
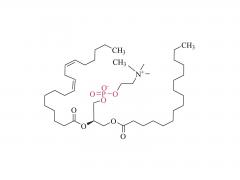
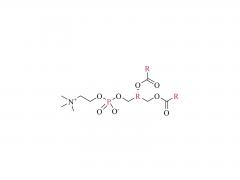
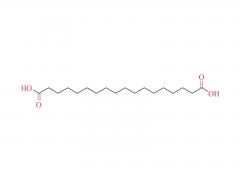
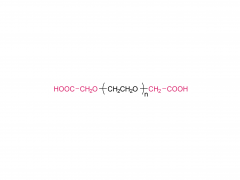
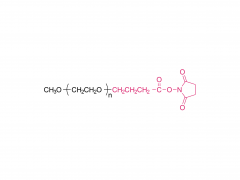
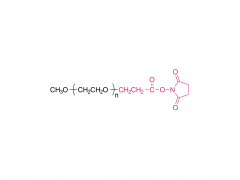
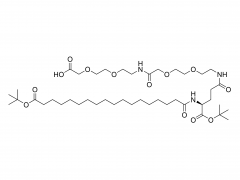
This golden autumn, SINOPEG ventured to Europe with its innovative products and solutions, making consecutive appearances at two premier international industry exhibitions within two months, engaging in deep exchanges with global pharmaceutical elites. CPHI Frankfurt 2025 (Frankfurt, Germany, Oct 28-30) As the world's largest and most influential professional event for the pharmaceutical industry, CPHI Frankfurt brought together industry elites from across the globe. In this grand gathering, SINOPEG attracted numerous visitors with its professional technical sharing. During the exhibition, we prominently showcased our latest achievements in areas such as in vivo CAR-T delivery, Antibody-Drug Conjugate (ADC) linkers, and block copolymers. We held in-depth discussions with multiple potential clients and partners from Europe, North America, and Asia! Many international clients praised our continuously improving R&D capabilities and product quality control system. TIDES Europe 2025 (Basel, Switzerland, Nov 11-13) Following closely, we moved to Switzerland to participate in TIDES Europe, an exhibition focused on oligonucleotides, peptides, mRNA therapies, and related fields. As one of the fastest-growing segments in biopharmaceuticals, TIDES provided us with a valuable platform for direct dialogue with global top experts. Here, we shared SINOPEG's technical expertise and project experience in fatty acid-modified side chains, LNP delivery material production, and related CMC services, attracting numerous biotechnology companies seeking reliable CDMO partners in this field. Through exchanges with industry benchmark companies, we further clarified our future technological development direction and innovation priorities. The successful participation in these two consecutive exhibitions allowed us to clearly grasp the development pulse of the global pharmaceutical industry chain. We deeply felt that CDMO enterprises from China are increasingly winning the trust of the global market, thanks to their continuously enhancing technical capabilities, flexible service models, and reliable quality systems. Two expeditions, two stages of growth. We brought back customer recognition, partner trust, and market insights – all of which will be invaluable assets for SINOPEG's future development! SINOPEG's journey on the international stage has just begun. We will continue to deepen our core technologies and expand our global footprint. Next year, we will appear on broader international stages, bringing more innovative solutions 'Made in China' to the global pharmaceutical industry. Stay tuned!
View More