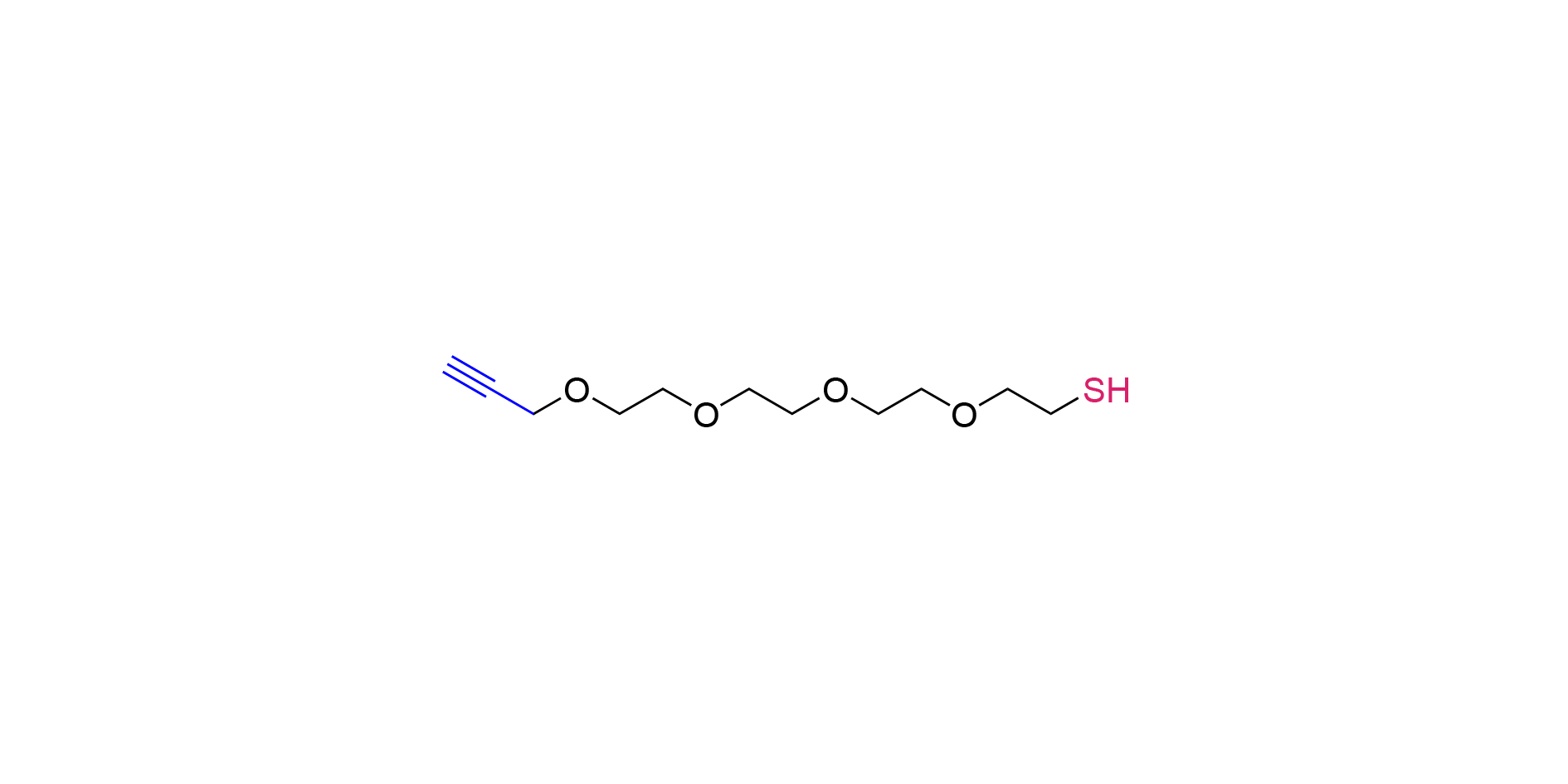
1. The basic nature
Molecular formula: C11H20O4S
Molecular weight: 248.3391
CAS number: 1347750-80-4
2. Chemical characteristics
Functional groups: Containing sulfhydryl (-SH) and propargyl functional groups.
Reactivity:
The sulfhydryl group is highly reactive and can participate in a variety of chemical reactions, such as addition reaction and substitution reaction.
The propargyl group can also participate in a variety of chemical reactions, especially the azide-acetylene click reaction (CuAAC) with the azide group catalyzed by copper to form a stable triazole bond.
3. Application fields
Biocoupling: SH-PEG4-Propargyl can be used as a biocoupling agent to link biological molecules (such as proteins, antibodies, etc.) with other compounds through chemical reactions.
Drug development: In the field of drug development, SH-PEG4-Propargyl may be used to prepare drug delivery systems, such as targeted drug carriers, to achieve targeted delivery and release of drugs by modifying drug molecules.
Materials science: In the field of materials science, SH-PEG4-Propargyl may be used for the preparation of nanomaterials or polymer composites with specific functions.
Related recommendations:
| Name | CAS |
|---|---|
| H2N-PEG6-OH | 39160-70-8 |
| H2N-PEG7-OH | 1425973-14-3 |
| H2N-PEG8-OH | 352439-37-3 |
| PA-PEG6-N3 | 361189-66-4 |
| PA-PEG8-N3 | 1214319-92-2 |
| PA-PEG9-N3 | 1670249-37-2 |
| N3-PEG6-OH | 86770-69-6 |
| N3-PEG7-OH | 1274892-60-2 |
| N3-PEG8-OH | 352439-36-2 |
| N3-PEG6-NH2 | 957486-82-7 |
| N3-PEG7-NH2 | 1333154-77-0 |
| N3-PEG8-NH2 | 857891-82-8 |
| HO-PEG6-CO-OtBu | 361189-64-2 |
| HO-PEG8-CO-OtBu | 1334177-84-2 |
| HO-PEG10-CO-OtBu | 778596-26-2 |
| Fmoc-NH-PEG5-CH2COOH | 635287-26-2 |
| Fmoc-NH-PEG6-CH2COOH | 437655-96-4 |
| Fmoc-NH-PEG7-CH2COOH | 152074-20-9 |
| Boc-NH-PEG7-NH2 | 206265-98-7 |
| Boc-NH-PEG9-NH2 | 890091-43-7 |
| Boc-NH-PEG11-NH2 | 890091-42-6 |





















