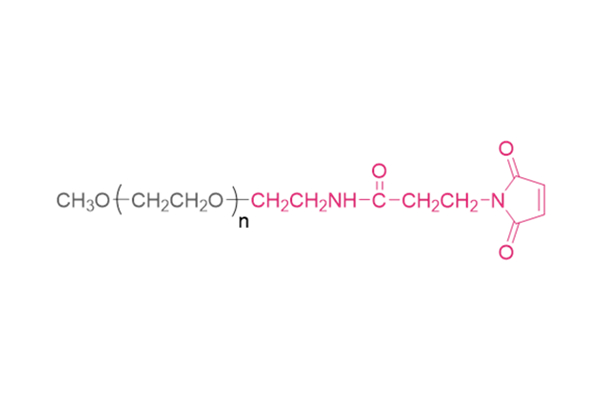
An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair
November 17,2020.
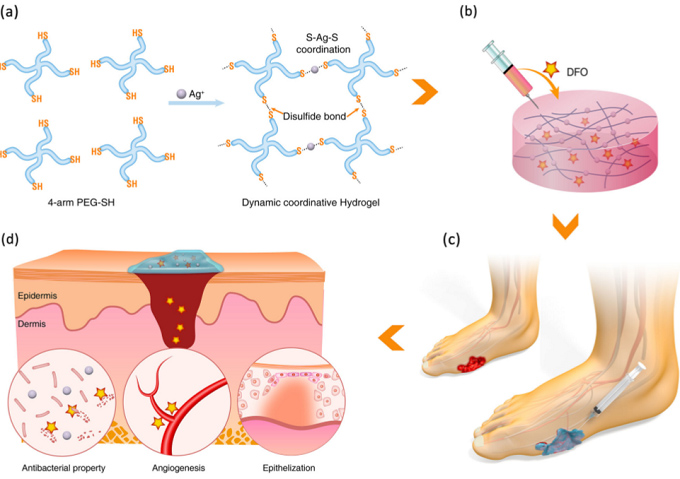
Diabetes can lead to nonhealing chronic ulcers over tendons, bones, and joints, and such conditions have to date led to more than 20 million patients suffering a single leg amputation. It is believed that the number of patients who will require lower limb amputation annually will double by 20301. The primary cause of the dreaded and chronic diabetic ulcer complication is impaired vessel formation, particularly microvasculature formation, which is critical for the delivery of oxygen, nutrients, and growth factors, all of which are needed for wound healing, especially in the early stages. Without sufficient angiogenesis (the formation of capillary blood vessel networks), high levels of glucose accumulate at the wound site, leading to ischemia and tissue necrosis. Thus, the reestablishment of the vascular network of diabetic wounds in the early stages of healing is essential to prevent wound expansion and ulcer formation in diabetic patients. In this article, they report an injectable, self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic wound regeneration. The hydrogel (referred to as Ag-SH-PEG) was simply prepared using coordinative crosslinking of multi-arm thiolated polyethylene glycol (SH-PEG) with silver nitrate (AgNO3) (Scheme 1a). Due to the dynamic and reversible nature of the Ag–S coordination bond, the resultant coordinative hydrogel featured self-healing properties after repeated rupture and injectable properties when applied through a medical needle. Such self-healing and injectable properties are particularly appealing for skin wound repair because they help reduce gel fragmentation and integrate ruptured gels at the target site, even after external mechanical destruction, and hence can continuously support skin wound healing. Moreover, the hydrogel network gradually releases antibacterial silver ions, which are highly attractive for use in susceptible open diabetic skin wounds. Due to the incorporation of an angiogenic drug, desferrioxamine (DFO), into the coordinative hydrogel, they finally obtained a multifunctional hydrogel that is manageable, resistant to external stress, antibacterial, and angiogenic (Scheme 1b). As an example, an irregular wound resulting from a foot ulcer is shown; in such cases, it is typically difficult for the vessels to grow in (Scheme 1c), which may be overcome in our proposed formulation. Furthermore, no self-healing hydrogels have been reported to include intrinsic structural properties that promote angiogenesis while simultaneously preventing bacterial infections. They anticipate that such unique multifunctional hydrogels will exhibit efficient anti-infective abilities, enhance angiogenic activity, and subsequently accelerate tissue healing in diabetic skin wound sites (Scheme 1d). Scheme 1 In summary, this article have described a multifunctional hydrogel scaffold with injectable, self-healing, antibacterial, and angiogenic properties for diab...
View More