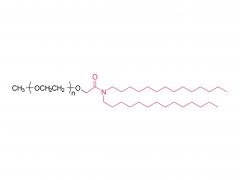
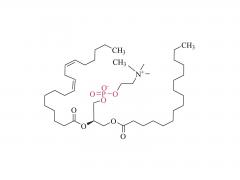
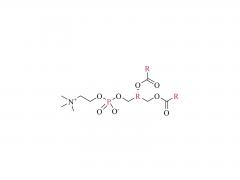
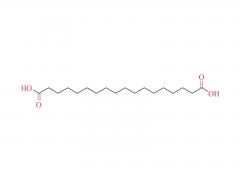
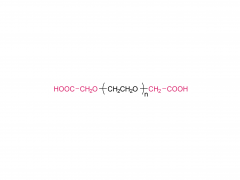
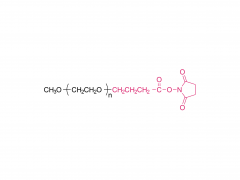
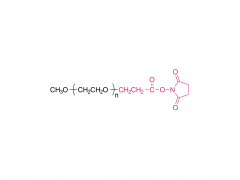
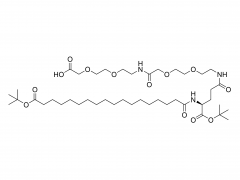
We are delighted to invite you to the prestigious CPHI CHINA, where SINOPEG will be showcasing our latest innovations and products. The event will be held at the Shanghai New International Expo Center from June 19th to 21st, 2024. CPHI CHINA is a leading platform for the pharmaceutical industry, bringing together top professionals, suppliers, and manufacturers from around the world. At our booth W4G31, you will have the opportunity to witness firsthand the cutting-edge solutions and advancements that SINOPEG has to offer. Our dedicated team will be available to provide detailed information, answer any queries, and discuss potential partnerships. We cordially invite you to join us at the SINOPEG booth W4G31 at CPHI CHINA and be part of this exciting event. Your presence and engagement will contribute significantly to the success of the exhibition. We look forward to meeting you and exploring potential synergies. Please feel free to contact us if you require any further information or to schedule a meeting during the event. We can be reached at sales@sinopeg.com. Thank you for considering our invitation. We eagerly anticipate your positive response and the opportunity to connect at CPHI CHINA.
View More