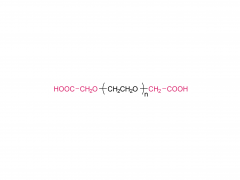
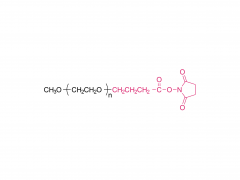
Molecules. 2017 Jul 15;22(7):1190. doi: 10.3390/molecules22071190. Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length Abstract A set of lipopeptides was recently reported for their broad-spectrum antiviral activity against viruses belonging to the Paramyxoviridae family, including human parainfluenza virus type 3 and Nipah virus. Among them, the peptide with a 24-unit PEG linker connecting it to a cholesterol moiety (VG-PEG24-Chol) was found to be the best membrane fusion inhibitory peptide. Here, we evaluated the interaction of the same set of peptides with biomembrane model systems and isolated human peripheral blood mononuclear cells (PBMC). VG-PEG24-Chol showed the highest insertion rate and it was among the peptides that induced a larger change on the surface pressure of cholesterol rich membranes. This peptide also displayed a high affinity towards PBMC membranes. These data provide new information about the dynamics of peptide-membrane interactions of a specific group of antiviral peptides, known for their potential as multipotent paramyxovirus antivirals. Keywords: antiviral; cholesterol; membranes; paramyxoviruses; peptides. PEG Linker: Various Kingds And Grades Of Such Monodispersed Are Readily Avaliable| SINOPEG
View More